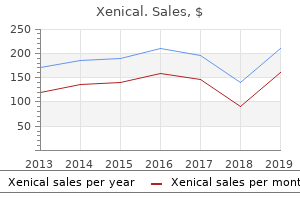
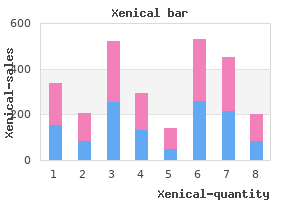
Xenical
"Discount 60mg xenical visa, weight loss with hypothyroidism".
By: S. Mine-Boss, M.A., M.D.
Associate Professor, California Health Sciences University
Selective exclusion is a variation of the chromatin accessibility mannequin rapid 60 weight loss pills buy xenical master card, by which PcG complexes might screen proteins on the basis of measurement weight loss in face purchase generic xenical canada, shape weight loss pills vitamin world buy xenical 60 mg on-line, or some other quality. McCall and Bender (one hundred ten) have proposed a number of different mechanisms of selective exclusion that may clarify these observations. Alternatively, PcG complexes might themselves immediately exclude binding by some other proteins on the basis of measurement or shape. The distinguishing feature of all these variations of the selective exclusion mannequin is that they suggest comparatively refined modifications of chromatin construction in comparison with the heterochromatin mannequin. This could involve blocking enhancer-promoter loop formation or in some other way stopping activators from interacting with the basal transcription machinery. In assist of this mannequin, PcG proteins which are tethered to reporter plasmids in mammalian tissue tradition cells have been shown to repress transcriptional activation in transient expression assays (112). This ability to repress varies with transcription factors which have totally different activation domains, suggesting an effect on activation however not access. These observations are additionally according to some variations of the selective exclusion mannequin, in that the effects on totally different activators could also be due to the exclusion of different accessory proteins which may be required for activation by some, however not different, transcription factors. Subnuclear compartmentalization It has been instructed that PcG-dependent silencing might sequester target genes in transcriptionally inert nuclear compartments (114). Once the silenced state of target genes is established throughout embryogenesis, it must be maintained through many cycles of cell division. Mammalian PcG proteins are required for proliferation and activation of hematopoietic cells (9, seventy three, 119, one hundred twenty). Thus, PcG proteins appear to perform an evolutionarily historical transcriptional silencing perform that has been tailored for the regulation of different genes and/or different developmental functions in phylogenetically dispersed organisms. Pirrotta (1998) Polycombing the genome: PcG, trxG, and chromatin silencing, Cell ninety three, 333336. Like proteins, the polyglycine polymer is formed by condensation of the amino group of 1 amino acid and the carboxyl group of another amino acid. On the opposite hand, proteins have a defined and sophisticated sequence that features all 20 of the amino acids, however polyglycine is a homopolymer formed solely from glycine residues. Glycine is the best amino acid residue, having a hydrogen atom as its aspect chain. The comparatively small measurement of the glycine aspect chain confers greater conformational flexibility to the polyglycine backbone in contrast with the backbone of different amino acid polymers. Polyglycine may also characterize an uncommon submit-translational modification; as much as 34 glycyl items have been observed covalently sure to the g-carboxyl group of C-terminal glutamic acid residues in tubulin (3). Furthermore, the insert has a single orientation, whereas a fraction with identical cohesive ends inserts in both orientation with equal probability. Pairs of polylinkers can be found which have the identical set of sites, however in opposite orientation, to permit cloning the fragments in both orientation (i. Polymer A polymer is a big molecule, or macromolecule, composed of many copies of repeating items joined collectively to kind a long chain. Of explicit curiosity to the molecular biologist are biopolymers similar to proteins, nucleic acids, and carbohydrates. A diverse range of synthetic polymers has been developed, together with plastics, synthetic fibers, and paints. The lengthy chain, or backbone, is the constant or repeating a part of the polymer. Homopolymers are polymers by which the repeating items are chemically and stereochemically identical. Both the backbone and aspect chains of homopolymers are constant throughout the length of the polymer. Polyethylene glycol and polyacrylamide are homopolymers commonly used in molecular biology. The repeating monomer items of those two reagents are ethylene glycol and acrylamide, respectively. Schematic illustration of a polymer, displaying the repeating nature of the backbone (X). The aspect chains (Y) connected to the backbone are constant in homopolymers however variable in copolymers. The association or order of the repeating items in copolymers can be both sequential or random. Proteins and nucleic acids are examples of organic copolymers (biopolymers) which have a specific sequence.
General mechanism of action of peptide hormones that bind to weight loss 3 weeks purchase xenical us receptors and thereby alter the activity of adeny cyclase by way of heterotrimeric G-proteins weight loss pills 982 buy 60mg xenical with mastercard. The peptide hormone binds to weight loss pills webmd purchase xenical 120 mg without prescription the extracellular a part of the receptor embedded plasma membrane. This is transmitted to the abg heterotrimeric G-protein, which dissociates in the Gbg-dimer and the G subunit, which binds to adenylate cyclase and alters its activity accordingly. Methods of Isolation and Structure Determination the construction of a peptide hormone was first demonstrated by de Vigneaud in 1953, when he decided the construction of oxytocin and confirmed it by total chemical synthesis. The chemical construction of secretin was not decided till 1966, 60 years after its initial discovery. Because peptide hormones exist in such small concentrations, it was difficult then to isolate, purify, and analyze them. Each isoform consists of 21 amino acid residues, they usually have excessive sequence homology. As a result of the progress in gene know-how, the chemical buildings of numerous hormone receptors have now been decided. New strategies are being developed to search for new bioactive peptides that serve as ligands for such orphan receptors. Synthesis and Conformational Analysis After the isolation and construction dedication of a peptide hormone has been achieved, the peptide is usually chemically synthesized to confirm the construction. With the synthesized materials, structureactivity relationships can be explored to design drugs for treating people with peptide-hormone deficiencies or with malfunctioning peptides. The technique of combinatorial chemistry has recently made it potential to synthesize hundreds of thousands of compounds and to display them for activity rapidly and effectively (7). In addition, gene know-how has provided a method of producing human peptide sequences, such as insulin, in Escherichia coli, yeast, or different expression systems. These labeled compounds can then be used to investigate the interaction between ligand and receptor, in addition to to research the conformational changes that occur upon binding. Peptide Libraries Peptide libraries have been first introduced through the mid-Nineteen Eighties as a method to elucidate the main points of antibodyantigen recognition (1, 2). This was the first case by which a problem in molecular recognition was elucidated utilizing a synthetic library and is usually cited as a important improvement in the emergence of combinatorial libraries. The first era of synthetic peptide libraries was limited in measurement to a few hundred peptides, as a result of the necessity for every library member to be ready as a discrete compound. However, the mixture of mixture-synthesis strategies (14), dependable methods for identifying active species from complex mixtures (3, four), and advances in peptide sequencing strategies rapidly led to the routine preparation and screening of libraries containing hundreds of thousands of distinct peptide sequences. Several reviews of ten-residue peptide libraries containing more than 1012 peptides have even appeared (5, 6). When coupled with an growing availability of unnatural amino acid building blocks, peptide libraries of this scale and complexity grew to become priceless tools for identifying novel peptide agents for a variety of functions (eg, see Refs. Soluble and solid-section peptide libraries continue to find functions in the identification of antigenic determinants. Many of these functions have employed normal assay codecs (eg, soluble activity or binding assays), requiring complex deconvolution or iterative synthesis and screening cycles to establish the active species from complex synthetic pools (see Combinatorial Synthesis). Because every bead carries a single compound in amounts approaching a number of nanomoles, assays can be performed instantly on the resin beads that detect interactions of bead-linked compound with a soluble target. Positive beads could also be selected manually, or more sophisticated instrumentation could also be employed to detect and isolate active beads (17). In an off-bead assay, the peptide is launched from the resin bead, but proximity to the bead is maintained by way of use of a diffusion-limiting matrix or a positional array. For instance, distribution of the library and a test cell line inside a delicate agar matrix, followed by partial peptide release, leads to the formation of locally excessive concentrations of peptide in the agar surrounding every bead. Zones of decreased cell growth (or any other reporting mechanism such as colour change) surrounding single beads are used to establish peptides having the specified activity. Beads producing a zone of activity are removed and analyzed by normal Edman Degradation or mass spectrometric evaluation to decide the sequences of the active peptides. This method has been used for identifying peptide ligands for G-protein-coupled receptors (20), in addition to cytotoxic peptides that may have application as anticancer agents (19).
Burren Myrtle (Bilberry). Xenical.
- Are there safety concerns?
- How does Bilberry work?
- Dosing considerations for Bilberry.
- Are there any interactions with medications?
- What is Bilberry?
- Chest pain (angina), varicose veins, cataracts, hardening of the arteries (atherosclerosis), diabetes, arthritis (osteoarthritis), gout, skin problems, hemorrhoids, urinary tract problems, chronic fatigue syndrome, and other conditions.
- Lesions in the eye (retina) in people with diabetes or high blood pressure.
- Improving night vision.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96235
The capability to weight loss pills mens health xenical 60mg low cost work in these three different ways are vital to weight loss cleanse cheap 60 mg xenical the practical diversity of G-protein motion weight loss pills and high blood pressure purchase xenical 60mg fast delivery. Guanidination Guanidination is a method for chemical modification of amino teams in proteins. The response is comparatively slow and proceeds primarily with the e-amino teams of lysine residues, converting them to homoarginine, a lot much less with a-amino teams. The operate of the modified protein is usually affected only if the amino group modified intimately participates in the operate. The response is followed by amino acid analysis, measuring the appearance of homoarginine and the decrease in lysine. The response is sort of particular to amino teams, however some aspect reactions happen with the thiol and imidazole teams of cysteine and histidine residues, respectively. Guanidination with O-methylisourea Almost all -amino teams of a protein are guanidinated (1) after remedy with 0. Guanidinium Salts Guanidinium chloride (GdmCl) is one of the most commonly used denaturants. The Gdm+ ion acts principally by competitively breaking intraprotein hydrogen bonds and forming hydrogen bonds with protein peptide bonds, although the Gdm+ ion has additionally a hydrophobic character and can work together on this method with protein nonpolar residues, principally aromatic teams (1). In contrast, the strongly stabilizing ion overcomes the denaturing capability of Gdm+. They are based mostly on a hydrophobic core of seven membrane-spanning a-helices that binds ligand on the extracellular face and binds G proteins on the cytoplasmic face. Alternatively, they may be regulated by ligand binding, by phosphorylation, by binding of yet other regulatory proteins, or by recruitment to their websites of motion when they or their anchors are phosphorylated. There are a number of methods in which the G-strand can type a very steady four-stranded construction. Indeed, the constructions of a number of telomeric sequences have been decided, showing the assorted forms of the G-quartet constructions. The G-quartet construction was shaped by the guanine residues being held collectively by a cyclic, head-to-tail network of hydrogen bonds and a metallic ion (eg,) positioned at the center. Another association of the tetra-stranded G-quartet is a fourfold symmetric motif in which the four Gstrands are in the parallel orientation. These two varieties differ of their construction, regulation, biochemical, and physicochemical properties (4-7). Those from sea urchins recognize speract and resact, small peptides that stimulate sperm motility and metabolism ((9),(10)). Intestinal cells comprise the receptor for the Escherichia coli heat-steady enterotoxin (guanylate cyclase C). The endogenous ligand for this intestinal receptor is a small peptide known as guanylin. They consist of an obvious extracellular area linked by a single transmembrane region to an intracellular area. The two subunits, alpha and beta, are proteins that, although completely different in size (from 70 to eighty two kDa) and sequence, are extremely associated (15). Two forms of beta subunits are at present identified, beta-1, which is expressed in lung and mind, and beta-2, which is extra abundant in kidney and liver. The most fascinating function of those subunits is that they bind a heme prosthetic group. Its importance has been emphasized by its position in blood circulation and cardiac muscle functioning (16, 17). This similarity suggests the existence of a standard ancestral purine nucleotide triphosphate cyclase. During Drosophila oogenesis, the gurken gene is transcribed in the cells of the germline. The activation of this major transmembrane receptor regulates the expression of a number of genes in the follicle cells, and thus initiates a series of events that lead to the right patterning of each the anteroposterior and the dorsoventral axis of the egg and embryo. It is homologous to the gene spitz of Drosophila, which acts as ligand of Egfr in the embryo and in imaginal discs. During Drosophila oogenesis, eggs are produced by way of the cooperation of the three completely different cell types that make up individual egg chambers; inside each egg chamber, an individual oocyte is connected at its anterior to a group of fifteen nurse cells, and this cluster of 16 cells is surrounded by a follicle cell epithelium (three). At this stage, the oocyte occupies solely a small a part of the amount of the egg chamber, and subsequently solely a restricted variety of follicle cells at the posterior of the egg chamber are involved with the oocyte membrane. In this group of follicle cells, the Egfr is activated by Gurken protein, which leads to the specification of those follicle cells as posterior cells.
Euploid A euploid cell is a cell that has the basic haploid number of chromosomes attribute of that species or any actual multiple of this haploid quantity weight loss while pregnant xenical 60 mg sale. Evolution Darwin outlined evolution within the Origin of Species as "descent with modification" (1) weight loss chart discount xenical 60 mg with mastercard. Although there have been various interpretations weight loss pills 70 buy xenical no prescription, modifications, and additions, the ideas of this definition proceed to be repeated as biology develops. In his principle, Darwin handled species as populations, and he acknowledged several features of populations that explain the process of evolution (1). In explicit, he noticed that among people having variation in characteristics, those having advantageous characteristics, or "health," can survive by producing extra offspring of their very own. De Vries proposed the mutation principle, which states that mutations appear all of a sudden in a population and that species will experience durations of speedy mutation (2). Natural choice then takes place so that people having advantageous characteristics can survive in a population, and the offspring have the identical kind of mutation, which turns into unfold in a population. The examine of molecular evolution is actually primarily based on the artificial principle of evolution. However, the development of molecular biology has introduced extra detailed and new information on molecular mechanisms for producing genetic variation, which have accelerated the development of evolutionary research. The genome tasks presently in progress will give insights to the evolutionary changes of genomes, structures, and features. De Vries (19011903) Die Mutationstheorie, Von Veit, Leipzig (19091910) "English translation", the Mutation Theory, trans. Evolutionary Distance Evolutionary distance is a distance by which evolutionary closeness or remoteness can be measured quantitatively. Evolutionary distance may be separated into two distinctive levels; one morphological; the opposite, molecular. In the case of evolutionary distance at the molecular degree, the number of nucleotide and amino acid substitutions, as well as immunological cross-reactions, are the most popular measures for evolutionary distance. The number of nucleotide substitutions is estimated by making pairwise comparison of nucleotide sequences and correcting for multiple substitutions at the same web site (see). For correction of multiple substitutions, one needs a mannequin of nucleotide substitution; for example, the one-parameter technique invented by Jukes and Cantor (1) assumed that the rates of nucleotide substitutions between all potential pairs of various nucleotides are equal to each other. In this case, the nucleotide substitution is estimated by the equation, where p is the fraction of nucleotide differences. In this way, 4-parameter technique and the six-parameter strategies had been also developed. The number of amino acid substitutions can also be used as a measure of evolutionary distance. Under the assumption that the substitution rates between any pair of amino acids are equal, the number of amino acid substitutions can be given by formulation Ka = ln(1p), where p represents the fraction of amino acid differences. It is well known, nonetheless, that the substitution rate between a pair of similar amino acids is far greater than that between a pair of nonsimilar amino acids. Although Dayhoff (3) developed the algorithm to estimate the number of amino acid substitutions bearing in mind the similarity matrix of amino acids, the formulation given was very difficult. To decrease this, Kimura (2) invented an empirical formulation by adding one simple term. The measurement of immunological distance makes use of as a measurement the intensity of immunological crossreaction between antigens and antisera that had been prepared from completely different species (see). It has now become less popular, nonetheless, because the numbers of amino acid or nucleotide substitutions include rather more quantitative information. Gojobori; "Evolutionary Distance" in (on-line), posting date: January 15, 2002, by T. Evolutionary Rate In the research of molecular evolution, the rates of nucleotide or amino acid substitutions (per web site per year) are often used as the evolutionary rate (see Sequence Analysis). If the divergence time between sequences A and B, which is measured by years, is denoted T, Kn is the number of substitutions which have taken place throughout 2T. Thus, the speed of nucleotide substitution (vn) can be computed by the formulation vn=Kn /(2T).